Vaccination represents one of the greatest advancements in modern medicine since it has significantly reduced the global burden of infectious diseases.1 Immunisation efforts globally have saved at least 154 million lives, or the equivalent of six lives every minute of every year, over the past 50 years.1
Additionally, over the past five decades, vaccination against 14 major infectious diseases (including diphtheria, Haemophilus influenzae type B, hepatitis B, Japanese encephalitis, measles, meningitis A, pertussis, invasive pneumococcal disease, polio, rotavirus, rubella, tetanus, tuberculosis and yellow fever) has been pivotal in reducing global infant mortality by 40 percent, and by more than 50 percent in the African region.1
In Australia, most vaccines are delivered through the National Immunisation Program (NIP), which provides publicly funded vaccines to protect people across all stages of life, with a focus on those at greatest risk (including infants and children, Aboriginal and Torres Strait Islander peoples, older adults, pregnant women and individuals with medical risk conditions).2
The continued elimination of polio, measles and rubella under the NIP demonstrates the effectiveness of the program, and the value of strong surveillance and outbreak response systems.2 Expert guidance on the implementation and delivery of the NIP is provided by the Australian Technical Advisory Group on Immunisation (ATAGI).3
At a national level, building on the achievements of the National Immunisation Strategy 2019–2024, the National Immunisation Strategy 2025–2030 aims to strengthen coverage rates and address recent declines in vaccination uptake. NIS2025 The National Immunisation Strategy 2025-2030 highlights six priority areas 2:
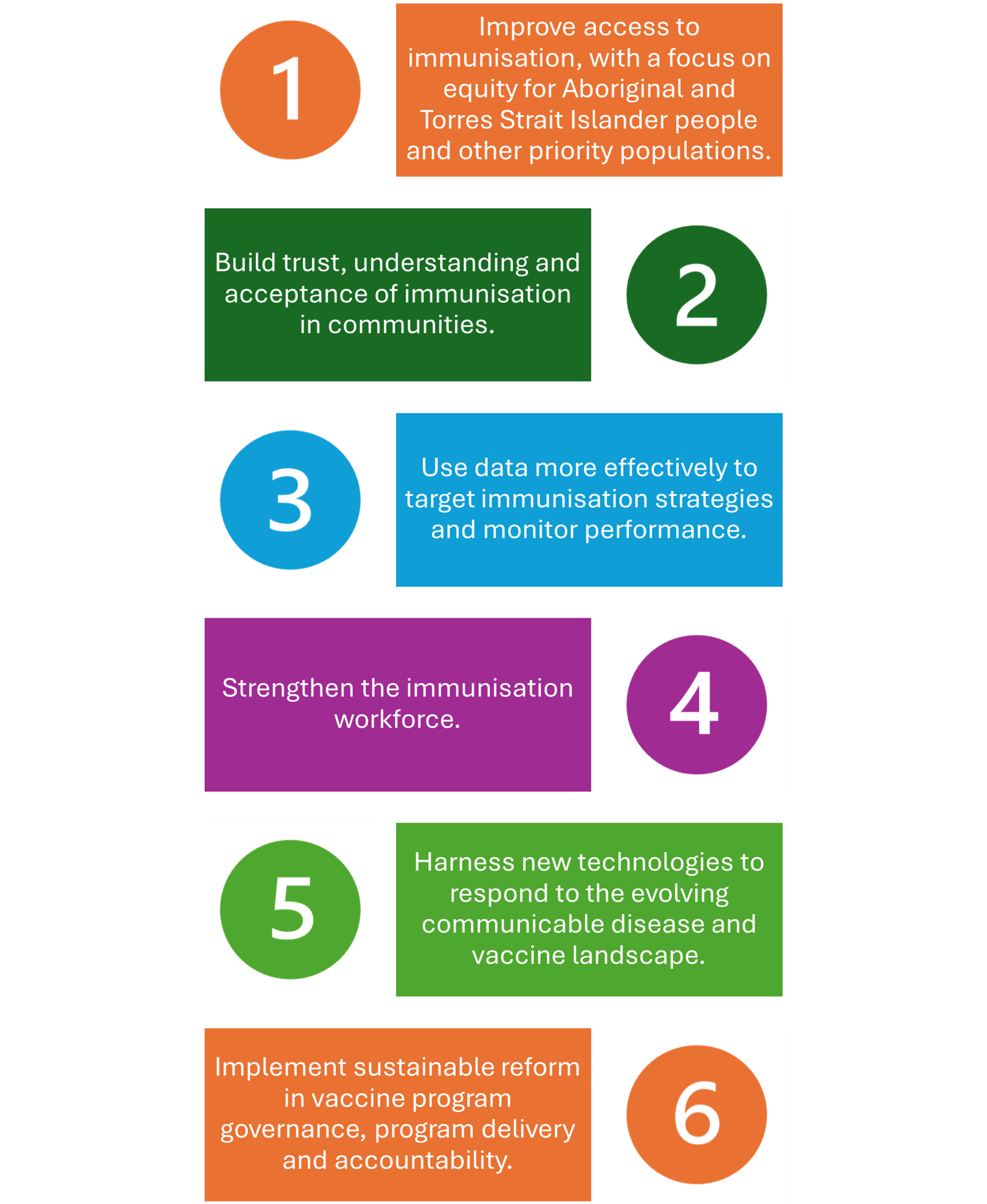
The COVID-19 public health response demonstrated Australia’s capacity to deliver large-scale immunisation rapidly and effectively, while also exposing inequities in vaccine access, knowledge and confidence among diverse communities.2 The National Immunisation Strategy 2025-2030 prioritises reducing disparities, improving community engagement and co-designing immunisation initiatives to enhance vaccine acceptance and trust in preventive health systems.2 Pharmacists play an important role in improving access, confidence and vaccination coverage across the nation.
Evolving scope of pharmacist vaccination
Community pharmacists are at the forefront of Australia’s primary healthcare system, with more than 5800 community pharmacies nationwide delivering essential health services and advice at approximately 462 million individual patient visits annually.4-5
Pharmacists are consistently recognised among Australia’s most trusted health professionals, alongside doctors and nurses.5 Surveys show 84 percent of adults trust the advice and guidance they receive from pharmacists, reinforcing the profession’s fundamental role in delivering accessible, patient-centred care.5
The scope of pharmacist vaccination in Australia is expanding rapidly, reflecting a move toward greater national consistency and broader clinical authority.4,6 Recent reforms, such as the National Immunisation Program Vaccinations in Pharmacy (NIPVIP), have significantly increased public access to vaccines through community pharmacies.4 Furthermore, the minimum patient age for pharmacist-administered vaccines has also been lowered across most states and territories, with some jurisdictions now permitting vaccinations for children as young as five years old.4
Notably, South Australia leads nationally by allowing pharmacists to administer any vaccine to any age group, provided it aligns with the Australian Immunisation Handbook (AIH) and professional scope.7 The integration of the NIPVIP program from 1 January 2024 allows eligible patients aged five years and above to access NIP-funded vaccines at no cost in participating pharmacies, marking a significant milestone in accessibility and public health equity, while recognising the professional and operational contributions of pharmacists.4,8-9
The range of vaccine-preventable diseases covered by pharmacist immunisation has expanded dramatically from a limited selection such as influenza, pertussis and measles, mumps and rubella (MMR) prior to 2020, to now include vaccines protecting against 21 diseases.4
This expanded scope requires pharmacists to be vigilant in managing varying vaccine brands, dosing schedules, contraindications and age-based eligibility.4 Maintaining accurate documentation, ensuring cold-chain compliance and promptly recording all vaccinations in the Australian Immunisation Register (AIR) are critical for patient safety and regulatory adherence.4
It is important to note that the scope of practice varies between jurisdictions, with differences in the vaccines permitted, eligible patient age groups, funding arrangements and vaccine supply pathways, meaning pharmacists must navigate differences in government-supplied stock, private vaccine procurement and reporting requirements. 4,6
These arrangements are updated regularly as legislation and public health priorities evolve. Ongoing efforts to achieve regulatory harmonisation, led by peak pharmacy bodies and the interim Australian Centre for Disease Control (CDC), aim to standardise pharmacist immunisation practices nationwide and reduce administrative variability between jurisdictions. 4,6,8-9
Pharmacists crucial contribution during the COVID-19 pandemic highlighted their ability to deliver large-scale vaccination services and effectively support public health responses, particularly in rural and remote communities where access to general practitioners(GPs) is limited.4 Pharmacist immunisers are now recognised as essential contributors to both routine and emergency immunisation programs, as well as travel health and community education initiatives.4
Strategic value of vaccination clinics
Vaccination clinics in community pharmacies play a critical role in strengthening Australia’s public health infrastructure.4 Community pharmacies are amongst the most accessible primary health care points, with extended opening hours, walk-in access and wide geographic reach making them ideal for vaccination services, especially for seasonal influenza, adult boosters, travel vaccinations and catch-up immunisations.4,10 Ability to get vaccinated in a pharmacy setting improves convenience and promotes equity to access, which is particularly beneficial to those in rural or underserved communities.4,10-11
Pharmacy involvement has been important in surge responses during COVID-19, seasonal influenza waves, 2022 Japanese encephalitis outbreak in Victoria and the ongoing outbreaks of pertussis across Australia.4 During the COVID-19 pandemic, pharmacists administered more than 11.6 million COVID-19 vaccine doses, accounting for around half of all vaccinations delivered nationally during peak rollout periods.4
Between March 1 2025 and October 5 2025, pharmacists administered approximately 2.6 million influenza vaccinations, representing roughly 29 percent of the total flu vaccine market during this period.12 These figures highlight pharmacists’ growing contribution to immunisation coverage and their vital role in reducing vaccine-preventable disease burden and strengthen herd immunity across the population.
Operational setup and workflow considerations
Clinic design and infection control
Pharmacy vaccination services must be conducted in a dedicated private consultation area that ensures patient privacy, confidentiality and safety.3 The space should be designed to meet professional and regulatory standards, allowing for efficient workflow and patient comfort.3,13
This area must be large enough to accommodate the pharmacist, the patient and any accompanying carer, as well as the equipment and documentation required for vaccine preparation and administration.3
The vaccine preparation and administration areas must be separated from general pharmacy activities to minimise distractions and contamination risk.13
For vaccines such as COVID-19 multi-dose vials, preparation should occur in a controlled area that supports proper labelling, expiry tracking and error prevention measures (e.g. visual reminders, checklists and flowcharts).13
The vaccine administration area should meet hygiene and accessibility requirements, providing enough space for patients with disabilities and suitable space to conduct cardiopulmonary resuscitation (CPR) in the event of an emergency.3,13
Hand-washing facilities or medical-grade sanitiser must be readily available, and the layout should allow for effective infection control, clear visibility and ease of movement during vaccination.3,13 Pharmacies must also have protocols and designated equipment for the safe disposal of sharps and unused vaccines, to prevent needle-stick injuries and ensure compliance with infection control standards.3,13
A post-immunisation observation area should be available with adequate seating and space for patients to remain under observation for at least 15 minutes after vaccination.3,13
An in-date anaphylaxis response kit must be available at all times, containing essential items such as adrenaline 1:1000 ampoules and/or adrenaline auto-injectors, syringes, cotton swabs, a stopwatch and laminated emergency response protocols for recognising and treating anaphylaxis.3,13-14 The emergency protocol should also be clearly displayed in the immunisation service delivery area for quick reference.3
Cold chain and vaccine storage
Maintaining the cold chain is an important element of safe and effective vaccine management within pharmacy-based immunisation services.3,14 Vaccines are sensitive biological products that must be stored between +2°C and +8°C to preserve potency and prevent degradation.3,14
Pharmacist immunisers must ensure that all vaccines are stored in purpose-built vaccine refrigerators or approved coolers that meet cold chain standards, as outlined in the National Vaccine Storage Guidelines: Strive for 5.3,14-15
Temperature monitoring must occur at least twice daily, with both minimum and maximum readings recorded.3,13 Vaccine refrigerators should be used exclusively for medicines and vaccines, located in a secure area accessible only to authorised personnel, and clearly labelled to prevent accidental power disconnection.3
Each pharmacy must have a written cold chain management protocol, which designates primary and backup staff responsible for vaccine storage, monitoring and breach response.3,13 Staff should complete regular Cold Chain Management training to ensure they understand how to manage deliveries, perform temperature checks, rotate stock by expiry date and implement a back-up plan during power outages.3,13
Following Strive for 5 guidance, pharmacists must have a clear cold chain breach procedure, including isolating affected vaccines, documenting the incident and contacting relevant health authorities or manufacturers for advice.3,13-15
Vaccines exposed to temperatures outside +2°C to +8°C must not be used or discarded until official advice is received.14-15 Proper documentation and adherence to established processes ensure both audit readiness and patient safety.3,13
Staff training requirements
In Australia, only authorised immunisers are permitted to administer vaccines. This includes medical practitioners, pharmacists, nurses, midwives and Aboriginal and Torres Strait Islander health practitioners who meet the required qualifications and legislative criteria.3
To practise as a pharmacist immuniser, pharmacists must hold current registration with the Pharmacy Board of Australia under Australian Health Practitioner Regulation Agency (AHPRA) and complete an accredited vaccination training program approved by the Australian Pharmacy Council and/or their state or territory Department of Health.3
These courses align with the Standards for the Accreditation of Programs to Support Pharmacist Administration of Vaccines.3 Pharmacists must also maintain a current first aid certificate (renewed every three years), CPR certificate (renewed annually) and complete anaphylaxis management training (renewed annually) such as that provided by the Australasian Society of Clinical Immunology and Allergy (ASCIA).3
The site to complete the training has changed in January 2025 and pharmacists are required to create an account on the new site to complete the training - https://traininghp.ascia.org.au/ .16
It is important to recognise that each state may have varying educational and training requirements for initial and ongoing immunisation provision, and it is important to be aware of the training requirements in your state.
In addition, pharmacist immunisers must hold professional indemnity insurance appropriate to their immunisation practice and comply with local legislative authorisation requirements.3
Best practice also requires the presence of another appropriately trained staff member, holding current CPR and first aid certification, to assist with post-vaccination observation and emergency response if required.13 Intern pharmacists can administer vaccines only in some states/territories, and only after completing approved training and under direct supervision of an authorised immuniser.
Pharmacists should ensure that all pharmacy staff are appropriately trained and prepared to support the delivery of immunisation services within the pharmacy workflow.3 Staff training should cover integration of vaccination services into daily operations, emergency procedures, workflow or layout adjustments, off-site service delivery and building relationships with consumers and other health professionals.3
All team members must understand their specific roles, responsibilities and relevant policies, including procedures for consumer communication, documentation, cultural safety and privacy.3 Training should also include recognising and responding to medical emergencies such as adverse events, anaphylaxis and vasovagal episodes, with all staff encouraged to complete anaphylaxis training.3 Pharmacist immunisers must maintain current certification in CPR, first aid and anaphylaxis management, and engage in continuing professional development (CPD) to sustain competence and confidence in vaccination services.3
Clinical procedures and protocols
Pharmacist immunisers must adhere to standardised clinical procedures and protocols to ensure vaccinations are delivered safely, ethically and in accordance with national and jurisdictional requirements.3
Pharmacists should develop a comprehensive policy and procedure manual for immunisation services in collaboration with the authorised immuniser or service provider.3 For Quality Care Pharmacy Program (QCPP) accredited pharmacies, the QCPP vaccination service template should be used as the foundation for creating this document to ensure alignment with accreditation and best practice standards.3
The policy and procedure manual for immunisation services should clearly outline the purpose, workflow, roles and responsibilities of all staff involved, supported by defined training schedules and protocols for safe vaccine management in line with Strive for 5 cold chain requirements.3
It must include detailed procedures for pre-vaccination screening, consent, emergency management (including anaphylaxis response), adverse event follow-up and work, health and safety, particularly regarding sharps handling and exposure prevention.3
Additionally, it should address waste management, documentation and reporting processes, ensuring that all vaccinations are recorded to the AIR and consumer privacy is protected under relevant legislation.3
The manual should also outline procedures for handling consumer enquiries and complaints, maintaining continuous quality improvement through incident reporting, audit schedules and regular review.3
Before administering any vaccine, pharmacists should complete a comprehensive pre-vaccination screening using an approved checklist to identify contraindications and precautions.3
Screening should include questions on allergies (particularly to vaccine components), bleeding or clotting disorders, pregnancy or breastfeeding status, acute or recent illness, immune suppression and previous adverse events following immunisation.3
Pharmacists should also review the patient’s immunisation history and consider recommendations from the AIH regarding co-administration of vaccines and management of temporary deferrals.3
Before administering a vaccination, pharmacist immunisers are required to advise of any out-of-pocket costs and availability of free government-funded vaccines.13
Legal and ethical considerations
Documentation
Accurate and thorough documentation is a fundamental requirement of pharmacist-led immunisation services.
Pharmacists must record key details for every vaccination, including the patient’s full name, date of birth, vaccine brand and batch number, dose, date and time of administration, injection site, immuniser’s name and the due date for any follow-up doses.3,13
Patient and primary healthcare provider contact details should also be included. 3
Records of consent, screening forms and vaccination details must be securely stored for at least seven years in accordance with privacy and data protection legislation, ensuring easy retrieval and confidentiality.3,13
Documentation should also include evidence of verbal or written consent, adherence to screening protocols and compliance with clinical and legal requirements outlined in the AIH and jurisdictional health regulations.3
Pharmacists must ensure all vaccinations are accurately documented, whether in written or digital form, to maintain complete and auditable patient records in line with professional and legislative requirements.14
While written documentation provides a physical record, digital recording, such as direct entry into the AIR, has numerous benefits including improved accuracy and reduced errors, enhanced accessibility, real-time data sharing between healthcare providers, improved continuity of care and reduced administrative burden.14
Wherever possible, pharmacists should prioritise digital documentation while securely storing written records to meet privacy and retention standards under the Privacy Act 1988. Proper documentation of all clinical decisions, consent and vaccine details supports quality assurance, professional accountability and continuity of care across the healthcare system.3
Reporting
Pharmacist immunisers are legally required to report all administered vaccines to the AIR, ideally within 24 hours of vaccination, using either the AIR website via Health Professional Online Services (HPOS) or integrated pharmacy software.13
This reporting ensures accurate national immunisation records and supports public health surveillance, policy and funding decisions. Pharmacists must be registered as AIR providers, with a unique provider number allowing access to vaccination histories and reporting capabilities.13
In addition, any adverse events following immunisation (AEFI) must be reported to the relevant state or territory health department and, where applicable, the Therapeutic Goods Administration (TGA) through the Adverse Event Management System (AEMS) or by submitting the National AEFI Reporting Form.3,13
Compliance
Compliance with immunisation legislation and professional standards is essential for safe and authorised vaccine delivery in community pharmacy.3
Pharmacies must be registered as immunisation providers with both the state or territory Department of Health and the AIR, ensuring that all immunisers practising on-site are appropriately authorised under jurisdictional regulations.3,13
Pharmacists must maintain access to up-to-date clinical references, including the AIH, National Vaccine Storage Guidelines: Strive for 5, and relevant state-specific vaccination program guidelines.3,13
The pharmacist-in-charge must also ensure adherence to protocols for vaccine storage, cold chain maintenance, anaphylaxis response and emergency procedures.3,13 Ongoing staff training, audit schedules and documentation of all policies and incidents further demonstrate compliance and support the delivery of a safe, high-quality immunisation service aligned with PSA and QCPP standards.3
Quality assurance and continuous improvement
Pharmacies should conduct regular internal audits to assess documentation accuracy (including consent forms, AIR submissions and vaccine batch details), verify compliance with cold chain protocols, ensure staff maintain current CPR, first aid and immunisation certifications and test emergency preparedness through mock anaphylaxis drills and refresher sessions, documenting all findings and corrective actions.3
Continuous improvement also relies on patient feedback, gathered through short digital or SMS surveys to evaluate satisfaction, communication, wait times and consent procedures.
Obtaining patient feedback and loyalty
Pharmacies should appoint a clinical lead immuniser responsible for maintaining policies, overseeing staff competency, managing incident reporting and ensuring regular updates to align with the Australian Immunisation Handbook and state and territory legislation.3
This proactive approach strengthens patient safety, regulatory compliance and public confidence in pharmacist-led vaccination services.
Financial considerations
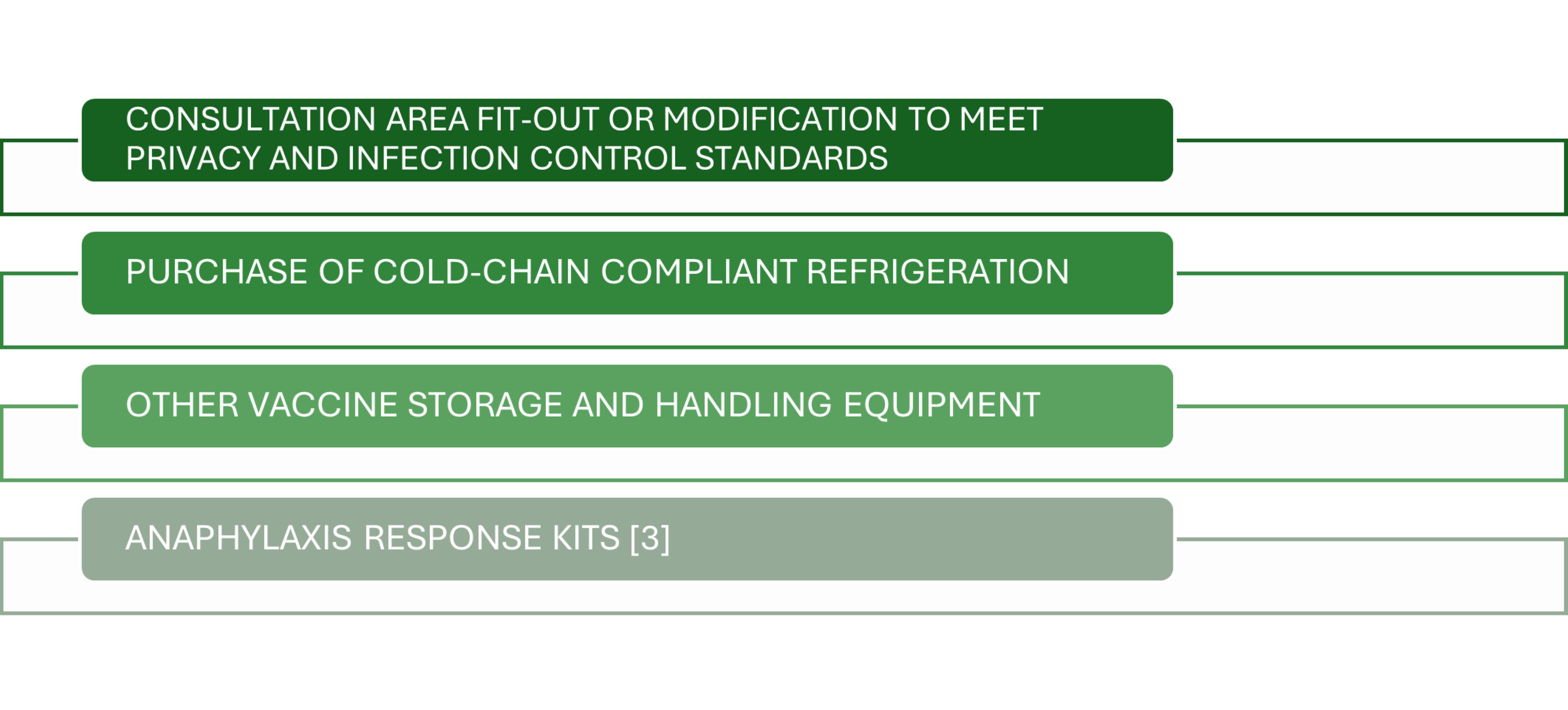
Establishing a vaccination clinic in a pharmacy involves several key cost components that must be carefully planned to ensure sustainability.3
Initial setup costs include:
Ongoing operational costs typically include:
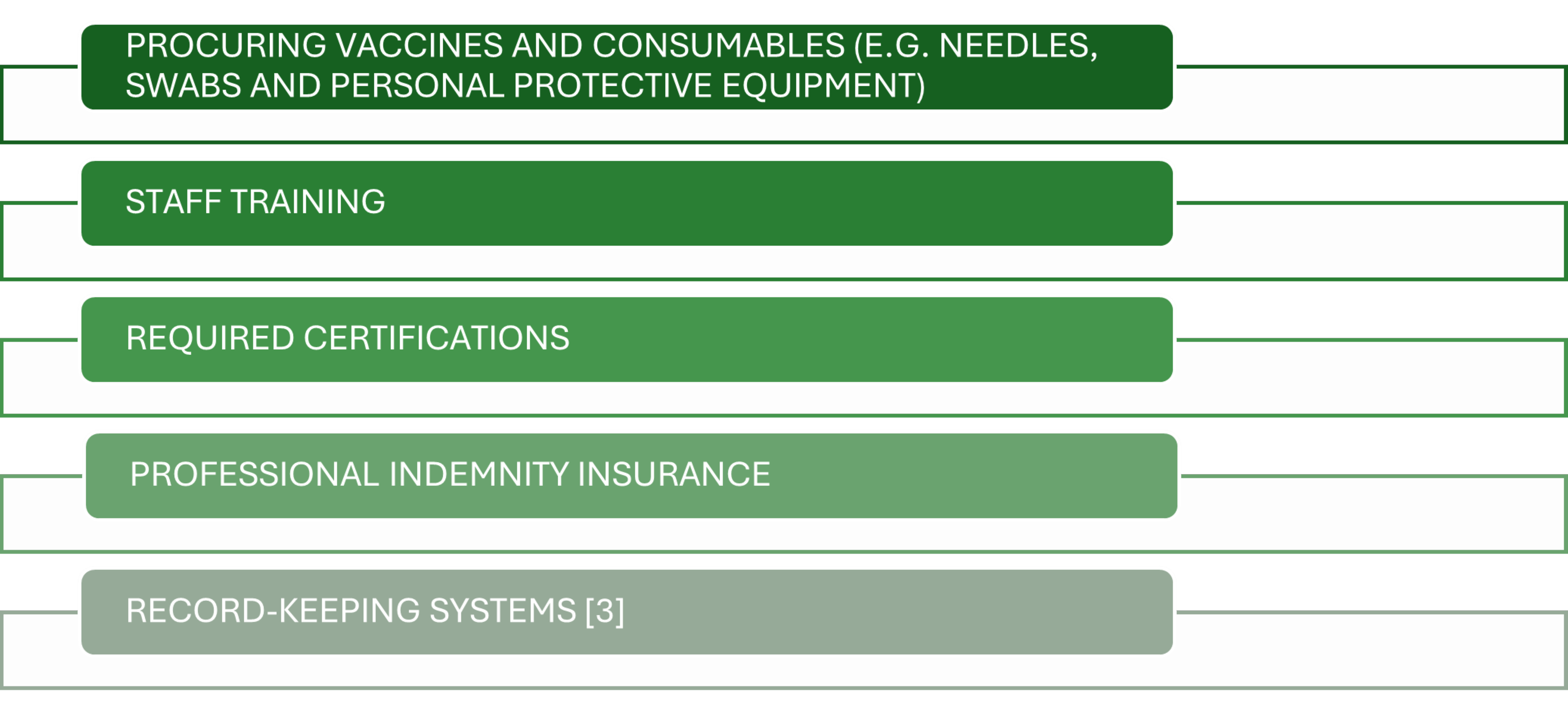
Digital tools for appointment scheduling and AIR reporting may also attract subscription fees. Pharmacists should also budget for marketing campaigns and community engagement to build patient awareness. These cost components vary based on state regulations and service scale but can typically range between AUD8,000–AUD15,000 for setup.
Revenue streams
Pharmacy vaccination clinics generate income through multiple streams, combining both private and government-funded models. Under the NIPVIP, pharmacies receive per-dose administration payments for eligible NIP-funded vaccines (as of 1 July 2025, AUD20.05 per NIP or COVID-19 vaccination), improving accessibility for patients while supporting business viability.8
Additional revenue is derived from privately funded vaccinations, such as influenza, travel and occupational vaccines, for which pharmacies can charge both consultation and administration fees.8
Indirect financial benefits also accrue through increased store traffic, improved medication adherence and greater opportunities for over-the-counter sales and health service referrals. Additionally, broader patient engagement often increases prescription and over-the-counter sales, as vaccinated customers tend to revisit for ongoing health needs. Furthermore, leveraging digital bookings and workplace partnerships helps sustain consistent patient flow across seasons.
Pricing and margins
Pricing should reflect both market competitiveness and the true cost of service delivery.3
Many routine vaccines, including those for infants, children, adolescents and adults, are free for eligible individuals through the NIP. The vaccines themselves are provided free by the government for eligible patients and pharmacies receive a service fee (AUD20.05) from the government for each vaccine administered.8
The price for non-NIP vaccines varies by pharmacy, but patients can expect to pay for both the vaccine and the administration fee. For example, some pharmacies may charge around AUD59.95 for a whooping cough (pertussis) vaccine, while others may charge around AUD20 for an influenza shot and AUD43 for a whooping cough (pertussis) vaccine if not government funded.
The whooping cough (pertussis) vaccine is only available as a combination vaccine (protecting against diseases such as diphtheria and tetanus).14
Margins are influenced by vaccine wholesale prices, supplier contracts and patient throughput. Pharmacies can optimise profitability by negotiating bulk vaccine purchasing arrangements, integrating efficient appointment systems to reduce staff downtime and maximising participation in funded programs such as NIPVIP.
Transparent pricing, combined with clear communication about value-added care (eg safety, convenience and professional expertise), enhances patient trust and repeat visits.
Return on investments – case study
A suburban Queensland pharmacy invested approximately AUD12 000 to launch a travel and occupational vaccination clinic offering hepatitis A, hepatitis B, typhoid and whooping cough vaccines.
In the first year, 950 vaccines were administered at an average fee of AUD75, with vaccine costs averaging AUD38 each. This generated roughly AUD80 000 in revenue and an estimated gross profit of AUD35 000, recovering the initial investment within eight months. Additional benefits included a 15 percent increase in prescription sales and expanded relationships with local employers for workplace vaccinations.
This case scenario highlights how diversified vaccine services can deliver rapid ROI while strengthening pharmacy visibility and community health outcomes.
Marketing and patient engagement
There are several strategies pharmacies can use to effectively reach priority groups and encourage patient attendance at vaccination clinics, including:
- In-pharmacy promotion: Use of posters, digital screens and counter displays in high traffic areas to highlight available vaccines and eligibility (e.g. seasonal influenza, human papillomavirus, shingles). Additionally, pharmacists and pharmacy staff should proactively initiate conversations about vaccinations during dispensing and medication reviews.
- Targeted patient reminders: Send SMS or email reminders to patients that are due for their routine vaccinations (eg influenza, pneumococcal or booster doses), using appropriate pharmacy software or loyalty program data. Where possible, link these reminders with booking links to allow patients to schedule their appointments online.
- Digital marketing: Promote vaccination services via various digital platforms including on the pharmacy’s website, social media and Google business profile. It is important to ensure that clinic hours, pricing and online booking is included. Information provided should be evidence-based and credible to build trust and encourage vaccination.
- Community engagement: Partner with local businesses, age care facilities, schools or workplaces to offer group vaccination days or on-site clinics. Participation in local public health events or community talks can also raise awareness of pharmacy vaccination services.
- Collaborative healthcare promotion: Pharmacies can work closely with nearby GP’s, allied health professionals and local health networks to coordinate referrals for eligible patients, thereby ensuring continuity of care and improving public health outcomes.
- Convenience and incentives: Offer extended hours, walk-in options and packaged health services (eg flu shot and medicine review) to attract patients seeking accessible and efficient care.
Conclusion
Pharmacy-based vaccination clinics are now a cornerstone of Australia’s immunisation landscape, offering convenient, accessible and trusted care to patients, while supporting national public health goals.
When implemented effectively, pharmacy vaccination services enhance vaccination coverage, improve community protection and strengthen the pharmacy’s reputation as a frontline health destination. Success depends on the integration of strong clinical governance, adherence to regulatory standards, careful financial planning, smart marketing and seamless operational workflows that ensure safety, efficiency and patient satisfaction.
For pharmacies, long-term success comes from aligning quality care with business sustainability, and this includes investing in accredited staff training, maintaining cold chain integrity, ensuring timely AIR reporting and engaging patients through targeted marketing and clear communication.
Participation in programs such as the NIPVIP, combined with transparent pricing and efficient workflow design, can drive both public health impact and financial growth. When guided by clinical excellence and a strong focus on patient-centred care, pharmacy-based vaccination clinics achieve tangible benefits for patients, strengthen community health outcomes and enhance the overall value of pharmacy practice.
Australia
Accreditation Number: A2512AUP1
Accreditation expiry: 30/11/2027
Pharmacist Competencies: 1.3, 2.1, 2.2, 2.3, 3.1, 3.2, 3.5, 3.6, 4.4, 4.5, 4.6
This activity has been accredited for 1.0 hr of Group 1 CPD (or 1.0 CPD credit) suitable for inclusion in an individual pharmacist’s CPD plan which can be converted to 1.0 hr of Group 2 CPD (or 2.0 CPD credits) upon successful completion of relevant assessment activities.
New Zealand
This article aims to equip you with the tools necessary to meet recertification requirements and actively contribute to the growth of
your professional knowledge and skills.
Effectively contribute to your annual recertification by utilising this content to document diverse learning activities, regardless of whether this topic was included in your professional development plan.
References
- World Health Organisation. Global immunization efforts have saved at least 154 million lives over the past 50 years. Joint News Release. Geneva/New York. Seattle. 24 April 2024. Available from: https://www.who.int/news/item/24-04-2024-global-immunization-efforts-have-saved-at-least-154-million-lives-over-the-past-50-years [Date accessed 9 Oct 2025]
- Australian Government. Department of Health, Disability and Ageing. National Immunisation Strategy for Australia 2025-2030. 12 June 2025. Available from: https://www.health.gov.au/resources/publications/national-immunisation-strategy-for-australia-2025-2030?language=en [Date accessed 9 Oct 2025]
- Pharmaceutical Society of Australia. Practice Guidelines for pharmacists providing immunisation service. May 2020. Available from: https://www.ppaonline.com.au/wp-content/uploads/2021/07/PSA-Immunisation-Guidelines.pdf [Date accessed 10 Oct 2025]
- Hart B. The evolving role of Australian community pharmacists in vaccination: challenges and opportunities. Microbiology Australia.2024.;5(4):201–204. Available from: https://doi.org/10.1071/MA24055 [Date accessed 11 Oct 2025]
- The Pharmacy Guild of Australia. About the Guild. The Pharmacy Guild of Australia.2025 Available from: https://www.guild.org.au/about-us [Date accessed 11 Oct 2025]
- Pharmaceutical Society of Australia. “Time for action”: Australia’s CDC backs pharmacists full scope vaccination. 13 Jun 2025. Available from: https://www.psa.org.au/time-for-action-australias-cdc-backs-pharmacists-full-scope-vaccination/ [Date accessed 12 Oct 2025]
- Pharmaceutical Society of Australia. PSA welcomes SA’s nation-leading vaccination scope for pharmacists and calls on other states to follow. 6 Feb 2025. Available from: https://www.psa.org.au/psa-welcomes-sas-nation-leading-vaccination-scope-for-pharmacists-and-calls-on-other-states-to-follow/[Date accessed 12 Oct 2025]
- Pharmacy Programs Administrator (2024) National Immunisation Program Vaccinations in Pharmacy (NIPVIP) Program. PPA. Available from: https://www.ppaonline.com.au/programs/national-immunisation-program-vaccinations-in-pharmacy-program [Date accessed 14 Oct 2025]
- Pharmacy Guild. NIP Vaccinations in Pharmacy. Updated 23 Jan 2025. Available from: https://www.guild.org.au/programs/vaccination-services/nip-vaccinations-in-pharmacy [Date accessed 14 Oct 2025]
- The Pharmacy Guild of Australia. Vaccination Services. 2025. Available from: https://www.guild.org.au/programs/vaccination-services [Date accessed 16 Oct 2025]
- Giannellis C. National Immunisation Program expansion: Exploring the path to a thriving pharmacy vaccination service. Hummingbird Pharmaceutical Consultancy. 2023. Available from: https://vaxapp.com.au/wp-content/uploads/2023/10/NIP-expansion-Exploring-the-path-to-a-thriving-pharmacy-vaccination-service-A-guide-by-Hummingbird-PC-for-VaxApp.pdf [Date accessed 18 Oct 2025]
- Australian Government. Department of Health, Disability and Ageing. Influenza (flu) immunisation data – 1 March to 5 October 2022-2025. Available from: https://www.health.gov.au/resources/collections/influenza-flu-immunisation-data [Date accessed 20 Oct 2025]
- Victoria State Government. Department of Health. Victoria Pharmacy Authority. July 2023. Available from: https://www.health.vic.gov.au/immunisation/pharmacy-services-self-audit-tool-immunisation-site-readiness [Date accessed 21 Oct 2025]
- Australian Technical Advisory Group on Immunisation (ATAGI). Australian Immunisation Handbook. Canberra: Australian Government Department of Health and Aged Care; 2025. Available from: https://immunisationhandbook.health.gov.au/ [Date accessed 21 Oct 2025]
- Australian Government. Department of Health, Disability and Ageing. National Vaccine Storage Guidelines ‘Strive for 5’. Sept 2025. Available from: https://www.health.gov.au/resources/publications/national-vaccine-storage-guidelines-strive-for-5?language=en [>Date accessed 24 Oct 2025]
- Pharmacy Guild of Australia. Required Updates for Approved Immunising Pharmacists. Updated: 11 September 2025. Available from: https://www.guild.org.au/guild-branches/wa/immunisation/annual-updates [Date accessed 25 Oct 2025]










